Modeling and simulation have grown to be indispensable in drug development. Using modeling and simulation, existing data could be leveraged to supply important insights on product safety and effectiveness as associated with drug concentration. This may be used to inform medical trial design and predict trial outcomes. They may also help drug developers select appropriate doses for first in human numerous studies or perhaps in special populations, for example kidney and hepatic impairment or pediatric patients.
Together, the data and insights gleaned from modeling and simulation provide not just possibilities for any more effective drug development program, they also provide key support for marketing application submissions. Indeed, the Food and drug administration and EMA
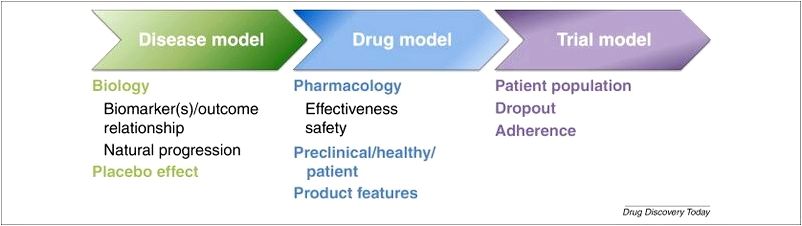
With modeling and simulation, your overall data can answer many important questions and save significant money and time. Model-based drug development includes using mathematical and record techniques to:
- Know how various dosing choices (e.g., dose, dose frequency, dosing duration) affect drug concentrations
- Clarify the connection between drug concentration and preferred or undesired pharmacodynamic responses
- Characterize the PK/PD variability of medication and help in comprehending the clinically relevant factors adding to variability
Modeling and Simulation Services
Nuventra comes with an expert team of pharmacometricians who build models tailored to steer your drug development program’s decisions. Included in these types of services, we can create a custom, model-based drug development plan which will facilitate selecting probably the most salient model for the compound and disease area.
Call us right now to find out how Nuventra’s modeling and simulation services will help you gain insights that move your clinical development program forward.
Resourse:https://www.nuventra.com/services/modeling-simulation/